Ethinamate
 | |
| Clinical data | |
|---|---|
| Trade names | Valmid, Valamin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 2.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.355 |
| Chemical and physical data | |
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ethinamate, marketed under the brand names Valmid and Valamin, is a central nervous system depressant of the carbamate drug class. It was formerly prescribed as a hypnotic for the short-term treatment of insomnia. The drug has a rapid onset of action, a short elimination half-life of around 2.5 hours, and a correspondingly brief duration of effect. Prolonged use may lead to tolerance and drug dependence, and efficacy typically diminishes after about a week of continuous use.[2]
Availability
[edit]Ethinamate is no longer available in the Netherlands, Canada, Belarus, Czech Republic, nor the United States,[3] having been replaced as an insomnia treatment by benzodiazepines and Z-drugs.[clarification needed][citation needed]
In the United States, ethinamate is now primarily remembered in connection with the toxicology reports on the death of Elvis Presley: it was listed among multiple sedatives detected in his system, and some forensic experts, including Cyril Wecht, later emphasized that polypharmacy was an important factor in the case.[4]
Toxicity and overdose
[edit]Ethinamate has a broader therapeutic index than barbiturates, and fatal overdose due to respiratory depression is uncommon.[citation needed] Overdose symptoms include CNS depression, low blood pressure, shallow breathing, loss of consciousness, and in severe cases, a comatose state. Hypersensitivity reactions may involve thrombocytopenia purpura, fever, and various skin rashes.[3]
Reported cases illustrate that very high doses (15–28 grams) can induce coma, but recovery is possible.[citation needed] Doses up to 1 gram are generally non-fatal, though they may cause prolonged sleep inertia or hangover-like effects. Treatment of overdose focuses on supportive care. Activated charcoal may reduce absorption if administered early (25–100 g for adults; 25–50 g in children under 12; 1 g/kg in infants), and hemodialysis can be considered in severe cases.[citation needed] Respiratory support may be necessary, similar to barbiturate management.
Adverse effects and pregnancy
[edit]Ethinamate's primary effects result from CNS depression, manifesting as slow heart rate, shallow breathing, and low blood pressure. Minor side effects at therapeutic doses include nausea, vomiting, gastrointestinal upset, and skin rash. Rare hypersensitivity reactions may involve thrombocytopenia purpura, fever, and rashes. Children are more likely than adults to experience paradoxical effects, so the drug was not indicated for individuals under 18.[citation needed]
The drug is classified as pregnancy Category C, and is not recommended during pregnancy or breastfeeding due to insufficient evidence regarding potential teratogenic effects. Animal studies suggest porphyrinogenic potential in vivo. [citation needed]
Chemistry and synthesis
[edit]The compound is synthesized by reacting acetylene with cyclohexanone to form 1-ethynylcyclohexanol. This intermediate is then converted into a carbamate through reaction with phosgene and ammonia. Small amounts of lithium or similar reagents may be used to facilitate the initial acetylene reaction.[5][6]
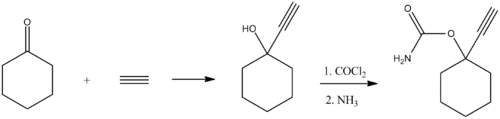
Ethinamate, whose molecular structure is known as 1-ethynylcyclohexanyneone carbamate is an odorless, white crystalline powder. It is typically formulated as 500 mg tablets for oral administration, with an adult dose of one tablet at bedtime; two tablets may be taken if necessary. Children are more prone to paradoxical effects and the drug is not indicated for individuals under 18.[citation needed]The compound is synthesized by reacting acetylene with cyclohexanone to form 1-ethynylcyclohexanol. This intermediate is then converted into a carbamate through reaction with phosgene and ammonia. Small amounts of lithium or similar reagents may be used to facilitate the initial acetylene reaction.[5][6]
After ingestion, ethinamate is metabolized to the active compound 4-hydroxyethinamate, which is eliminated in urine with a half-life of approximately 2.5 hours.[2]
References
[edit]- ^ Anvisa (31 March 2023). "RDC Nº 784 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b "Ethinamate (Valmid, Valamin): Overview". PubChem. Retrieved 2 May 2025.
- ^ a b Lowry WT, Garriot JC (1979). "Ethinamate". Forensic Toxicology: Controlled Substances and Dangerous Drugs. Boston, MA: Springer US. p. 215. ISBN 978-1-4684-3444-6.
- ^ Cole J, Thompson C (2 August 2017). "The Death of Elvis". Memphis Magazine. Memphis Magazine. Retrieved 4 September 2025.
- ^ a b US 2816910, Pfeiffer H, Junkman K, "Esters of carbamic acid and a method of making same", issued 17 December 1957, assigned to Schering AG
- ^ a b DE 1021843, Emde H, Grimme W, "Verfahren zur Herstellung des Allophanats des 1-AEthinylcyclohexanols-(1)", issued 2 January 1958, assigned to Rheinpreussen AG