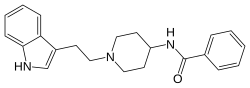
Indoramin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.659 |
| Chemical and physical data | |
| Formula | C22H25N3O |
| Molar mass | 347.462 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Indoramin (trade names Baratol and Doralese) is a piperidine antiadrenergic agent.
It is an alpha-1 selective adrenoceptor antagonist[1] with direct myocardial depression action; therefore, it results in no reflex tachycardia. It is also used in benign prostatic hyperplasia (BPH).[2]
It is commonly synthesized from tryptophol.[3]
Dosage
[edit]Indoramin is commonly prescribed as 20 mg tablets when used in BPH.[4]
Side Effects
[edit]Drowsiness, dizziness, dry mouth, nasal congestion, headache, fatigue, weight gain, hypotension, postural hypotension, depression, problems with ejaculation, diarrhoea, nausea, increased need to pass urine, and palpitations.[5]
Synthesis
[edit]Tryptamine and serotonin are naturally occurring indole ethylamino compounds with pronounced pharmacological activities. They have served as the inspiration for synthesis of numerous analogues.
One such study involved alkylation of 4-benzamidopyridine (2) with a bromoethyy compound (1) derived from tryptophol, to give a quaternary pyridinium salt (3); this intermediate was in turn hydrogenated with a Raney nickel catalyst to give indoramine.[6][7]
Product withdrawal
[edit]On May 31, 2013, the French National Agency for the Safety of Medicines and Health Products (ANSM) concluded that the benefit/risk ratio of this product was unfavorable and withdrew Vidora's marketing authorization and recalled its batches from the market on June 3, 2013.[8]
References
[edit]- ^ Pierce V, Shepperson NB, Todd MH, Waterfall JF (February 1986). "Investigation into the cardioregulatory properties of the alpha 1-adrenoceptor blocker indoramin". British Journal of Pharmacology. 87 (2): 433–441. doi:10.1111/j.1476-5381.1986.tb10834.x. PMC 1916533. PMID 3955309.
- ^ "Indoramin 20mg tablets". Medicines.org.uk. April 20, 2011. Archived from the original on July 25, 2022. Retrieved September 30, 2012.
- ^ Ullman's encyclopedia of Industrial Chemistry, Sixth Edition, 2002.
- ^ "Indoramin hydrochloride". National Health Service (UK). Retrieved September 30, 2012.
- ^ "Indoramin 20mg tablets". Medicines.org.uk. Retrieved February 7, 2018.
- ^ ZA 6803204, Archibald JL, Jackson JO; eidem, U.S. patent 3,527,761 (1969, 1970 both to Wyeth).
- ^ Archibald JL, Alps BJ, Cavalla JF, Jackson JL (November 1971). "Synthesis and hypotensive activity of benzamidopiperidylethylindoles". Journal of Medicinal Chemistry. 14 (11): 1054–1059. doi:10.1021/jm00293a009. PMID 5115203.
- ^ "Actualités". ANSM (in French). Retrieved 2023-04-17.
