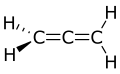| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Allene[1]
| |||
| Preferred IUPAC name
Propa-1,2-diene[2] | |||
| Systematic IUPAC name
Propadiene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730774 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.670 | ||
| EC Number |
| ||
| 860 | |||
| MeSH | Propadiene | ||
PubChem CID
|
|||
| UNII | |||
| UN number | 2200 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4 | |||
| Molar mass | 40.065 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Melting point | −136 °C (−213 °F; 137 K) | ||
| Boiling point | −34 °C (−29 °F; 239 K) | ||
| log P | 1.45 | ||
| Hazards | |||
| GHS labelling: | |||
 [3] [3]
| |||
| Danger | |||
| H220[3] | |||
| P210, P377, P381, P410+P403[3] | |||
| NFPA 704 (fire diamond) | |||
| Explosive limits | 13% | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propadiene (/proʊpəˈdaɪiːn/) or allene (/ˈæliːn/) is the organic compound with the formula H2C=C=CH2. It is the simplest allene, i.e. a compound with two adjacent carbon double bonds.[4] As a constituent of MAPP gas, it has been used as a fuel for specialized welding.
Production and equilibrium with methylacetylene
[edit]Propadiene exists in equilibrium with methylacetylene (propyne) and the mixture is sometimes called MAPD for methylacetylene-propadiene:
- H3C−C≡CH ⇌ H2C=C=CH2
for which Keq = 0.22 at 270 °C or 0.1 at 5 °C.
MAPD is produced as a side product, often an undesirable one, of dehydrogenation of propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene.[5]
Occurrence in Space
[edit]In 2019 it was announced that propadiene had been detected in the atmosphere of Saturn's moon Titan using the NASA Infrared Telescope Facility.[6] This was the first time that propadiene had been detected in space, and the second structural isomeric pair (paired with propyne) detected in Titan's atmosphere, after HCN-HNC.[7][8]
References
[edit]- ^ https://www.ebi.ac.uk/chebi/CHEBI:37601
- ^ Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. p. 375. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
The name allene, for CH2=C=CH2, is retained for general nomenclature only; substitution is allowed, but not by alkyl or any other group that extends the carbon chain, nor characteristic groups expressed by suffixes. The systematic name, propa-1,2-diene, is the preferred IUPAC name.
- ^ a b c Record of Allene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 17 November 2020.
- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "allenes". doi:10.1351/goldbook.A00238
- ^ Klaus Buckl, Andreas Meiswinkel "Propyne" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.m22_m01
- ^ Lombardo, Nicholas A; Nixon, Conor A; Greathouse, Thomas K; Bézard, Bruno; Jolly, Antoine; Vinatier, Sandrine; Teanby, Nicholas A; Richter, Matthew J; G Irwin, Patrick J; Coustenis, Athena; Flasar, F Michael (2019-08-20). "Detection of Propadiene on Titan". The Astrophysical Journal Letters. 881 (2): L33. arXiv:1908.07424. doi:10.3847/2041-8213/ab3860. ISSN 2041-8205.
- ^ Moreno, R.; Lellouch, E.; Lara, L. M.; Courtin, R.; Bockelée-Morvan, D.; Hartogh, P.; Rengel, M.; Biver, N.; Banaszkiewicz, M.; González, A. (December 2011). "First detection of hydrogen isocyanide (HNC) in Titan's atmosphere". Astronomy & Astrophysics. 536: L12. doi:10.1051/0004-6361/201118189. ISSN 0004-6361.
- ^ Hébrard, E.; Dobrijevic, M.; Loison, J. C.; Bergeat, A.; Hickson, K. M. (May 2012). "Neutral production of hydrogen isocyanide (HNC) and hydrogen cyanide (HCN) in Titan's upper atmosphere". Astronomy & Astrophysics. 541: A21. doi:10.1051/0004-6361/201218837. ISSN 0004-6361.