GABAA receptor agonist
| GABAA receptor agonist | |
|---|---|
| Drug class | |
 | |
| Class identifiers | |
| Synonyms | GABAA agonist |
| Use | Seizures, insomnia, hallucinogenic effects |
| Mechanism of action | GABAA receptor agonism |
| Biological target | GABAA receptor |
| Chemical class | GABA analogues and others |
| Legal status | |
A GABAA receptor agonist is a drug that acts as an orthosteric agonist of the GABAA receptor, the major signaling receptor of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[1][2][3]
The mechanism of action of GABAA receptor agonists is unlike that of GABAA positive allosteric modulators, including benzodiazepines, Z drugs, barbiturates, neurosteroids, and alcohol, which instead act via allosteric regulatory sites to potentiate GABAA receptor function.[4][3] GABAA receptor agonists have different effects from those of GABAA receptor positive allosteric modulators.[5][6][7][8]
Examples of GABAA receptor agonists include GABA itself, γ-amino-β-hydroxybutyric acid (GABOB), muscimol (found in Amanita muscaria mushrooms), gaboxadol (THIP), and progabide, among others.[1][2][3] High-efficacy GABAA receptor agonists have been found to produce sedative, hypnotic, anticonvulsant, and hallucinogenic effects, among others.[9][10][11][12] The structural requirements for GABAA receptor binding and activation have been found to be very strict, so relatively few high-efficacy GABAA receptor agonists are known.[2][13] No fully selective GABAA receptor agonists, for instance lacking any additional activity at the closely related GABAA-ρ and/or GABAB receptors, are currently known.[14]
GABAA receptor agonists are generally GABA analogues and derivatives.[1][2] Muscimol, a conformationally restrained analogue of GABA, was among the first GABAA receptor agonists to be identified.[15]
GABA
[edit]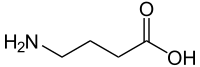
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system.[6][16][17] It is present in about 25 to 50% of neurons in the brain.[17] The neurotransmitter acts as a non-selective agonist of all three types of GABA receptors, including the GABAA receptor, GABAB receptor, and GABAA-rho receptor (GABAC receptor).[6][16] GABA is available as an over-the-counter supplement and is often taken for self-treatment of insomnia and anxiety.[18][19] However, GABA is highly susceptible to metabolism, has a very short elimination half-life, and is unable to cross the blood–brain barrier.[18][19] As such, the therapeutic benefits of exogenous GABA are questionable.[19]
GABOB
[edit]
γ-Amino-β-hydroxybutyric acid (GABOB) is a close endogenous analogue of GABA found in the human brain.[20] It acts as a dual GABAA and GABAB receptor agonist.[21] The drug has anticonvulsant effects and has been used in the treatment of epilepsy under brand names like Gamibetal in some countries throughout the world.[22][20][23]
Muscimol
[edit]

Muscimol is an alkaloid found in Amanita mushrooms such as Amanita muscaria (fly agaric).[10][15][24] It is a conformationally constrained derivative of GABA.[15][25] The drug is a highly potent GABAA receptor full agonist.[10][15] However, it is a selective or preferential agonist of extrasynaptic δ subunit-containing GABAA receptors.[10][26][27] The drug also acts as a potent GABAA-ρ receptor partial agonist and weak GABA reuptake inhibitor.[10][15][25] Muscimol is a sedative and hallucinogen.[10][28][29] Ibotenic acid, another alkaloid found in Amanita mushrooms, is a neurotoxin but functions as a prodrug of muscimol and has similar effects.[30][28][25] Muscimol has served as the base structure for development of many synthetic GABA system modulators, including GABA receptor modulators and GABA reuptake inhibitors.[5][31] The compound has been used recreationally as a hallucinogen and has become increasingly used at low doses for claimed therapeutic benefits, such as sleep improvement.[10][32][33][34]
Gaboxadol
[edit]
Gaboxadol (THIP) is a synthetic derivative of muscimol which acts as a potent GABAA receptor partial agonist.[7][8][11] However, it is a selective or preferential supramaximal agonist of extrasynaptic δ subunit-containing GABAA receptors.[2][7][9] The drug is also a potent GABAA-ρ receptor antagonist.[14] Gaboxadol has improved selectivy and drug-like properties compared to muscimol.[7][15][5][11] It has sedative, hypnotic, and, at high doses, hallucinogenic effects.[8][2][11] The drug was developed for treatment of insomnia and other conditions.[8][2][11] It was found to be effective in the treatment of insomnia, with advantageous properties compared to other hypnotics, such as increased slow wave sleep (deep sleep).[8][2][35] Gaboxadol completed phase 3 clinical trials for this indication.[36] However, it was discontinued for various reasons, most notably a narrow therapeutic index with high rates of hallucinogenic effects at supratherapeutic doses.[37][38][39]
Progabide
[edit]
Progabide is a GABA derivative which acts as a dual GABAA and GABAB receptor agonist.[12][40][41][42] It produces the more potent GABA receptor agonist progabide acid (SL-75102) as an active metabolite and is additionally a prodrug of gabamide and GABA.[12][41][43][42] The drug is used as an anticonvulsant under the brand name Gabrene in France.[44] The use of progabide has been limited by poor clinical effectiveness and incidence of liver toxicity.[45]
Picamilon
[edit]
Picamilon (N-nicotinoyl-GABA) is a GABA analogue, specifically a conjugate of GABA and nicotinic acid (niacin), which is used as a pharmaceutical drug and vasodilator in Russia for various indications.[46][47] In addition, it has emerged in supplements elsewhere in the world and is advertised and used as a purported nootropic (cognitive enhancer).[46][48] The drug crosses the blood–brain barrier and is hydrolyzed into GABA and nicotinic acid in animals, and hence is assumed to act as a centrally active and metabolism-resistant prodrug of these metabolites.[46][49] Picamilon itself has been found to be inactive at a large panel of targets, including the GABA receptors, GABA transporters, GABA transaminase, and calcium channels, which are all known targets for other GABA analogues.[46] The drug is relatively little-researched.[46]
Others
[edit]Other GABAA receptor agonists include 4-AHP,[50] dihydromuscimol, imidazole-4-acetic acid (IAA, IMA), isoguvacine, isonipecotic acid, methylglyoxal,[51] nefiracetam (DM-9384),[52] 4-PIOL, piperidine-4-sulfonic acid (P4S), quisqualamine,[53][54][55] thiomuscimol, and tolgabide (SL-81.0142), among others.[56][6][57] Some of these compounds, such as isoguvacine, isonipecotic acid, and P4S, are known to be unable to cross the blood–brain barrier.[58][7][59]
Indirect agonists
[edit]Indirect agonists of the GABAA receptor, as well as of the other GABA receptors, include GABA reuptake inhibitors like the anticonvulsant tiagabine (Gabitril) and GABA transaminase (GABA-T) inhibitors like the anticonvulsant vigabatrin (Sabril).[60][61][62][63]
See also
[edit]References
[edit]- ^ a b c Krogsgaard-Larsen P, Frølund B, Liljefors T (2002). "Specific GABA(A) agonists and partial agonists". Chem Rec. 2 (6): 419–430. doi:10.1002/tcr.10040. PMID 12469353.
- ^ a b c d e f g h Krogsgaard-Larsen P, Frølund B, Liljefors T (2006). "GABAA Agonists and Partial Agonists: THIP (Gaboxadol) as a Non-Opioid Analgesic and a Novel Type of Hypnotic1". GABA(A) agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Adv Pharmacol. Vol. 54. pp. 53–71. doi:10.1016/s1054-3589(06)54003-7. ISBN 978-0-12-032957-1. PMID 17175810.
- ^ a b c Ghit A, Assal D, Al-Shami AS, Hussein DE (August 2021). "GABAA receptors: structure, function, pharmacology, and related disorders". J Genet Eng Biotechnol. 19 (1) 123. doi:10.1186/s43141-021-00224-0. PMC 8380214. PMID 34417930.
- ^ Jembrek MJ, Vlainic J (2015). "GABA Receptors: Pharmacological Potential and Pitfalls". Curr Pharm Des. 21 (34): 4943–4959. doi:10.2174/1381612821666150914121624. PMID 26365137.
- ^ a b c Krogsgaard-Larsen P, Frølund B, Liljefors T, Ebert B (October 2004). "GABA(A) agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic". Biochem Pharmacol. 68 (8): 1573–1580. doi:10.1016/j.bcp.2004.06.040. PMID 15451401.
- ^ a b c d Frølund B, Ebert B, Kristiansen U, Liljefors T, Krogsgaard-Larsen P (August 2002). "GABA(A) receptor ligands and their therapeutic potentials". Curr Top Med Chem. 2 (8): 817–832. doi:10.2174/1568026023393525. PMID 12171573.
- ^ a b c d e Wafford KA, Ebert B (February 2006). "Gaboxadol--a new awakening in sleep". Curr Opin Pharmacol. 6 (1): 30–36. doi:10.1016/j.coph.2005.10.004. PMID 16368265.
- ^ a b c d e Sorbera, L.A.; Castaner, J.; Silvestre, J.S. (2004). "Gaboxadol". Drugs of the Future. 29 (5): 0449. doi:10.1358/dof.2004.029.05.803754. Retrieved 19 June 2025.
- ^ a b Brickley SG, Mody I (January 2012). "Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease". Neuron. 73 (1): 23–34. doi:10.1016/j.neuron.2011.12.012. PMC 3399243. PMID 22243744.
- ^ a b c d e f g Rivera-Illanes D, Recabarren-Gajardo G (September 2024). "Classics in Chemical Neuroscience: Muscimol". ACS Chem Neurosci. 15 (18): 3257–3269. doi:10.1021/acschemneuro.4c00304. PMID 39254100.
- ^ a b c d e Morris H (August 2013). "Gaboxadol". Harper's Magazine. Retrieved 2014-11-20.
- ^ a b c Bergmann KJ (1985). "Progabide: a new GABA-mimetic agent in clinical use". Clin Neuropharmacol. 8 (1): 13–26. doi:10.1097/00002826-198503000-00002. PMID 2983890.
Progabide, a new synthetic compound defined as the Schiff base of gamma-aminobutyramide and a substituted benzophenone, has been developed. Well absorbed, and relatively free of toxicity, it is both a direct GABA receptor agonist as well as an exogenous source of GABA.
- ^ Krogsgaard-Larsen, Povl (2018). "THIP/Gaboxadol, a Unique GABA Agonist". Reference Module in Biomedical Sciences. Elsevier. doi:10.1016/b978-0-12-801238-3.97290-8. ISBN 978-0-12-801238-3. Retrieved 7 October 2025.
- ^ a b Johnston GA (2005). "GABA(A) receptor channel pharmacology". Curr Pharm Des. 11 (15): 1867–1885. doi:10.2174/1381612054021024. PMID 15974965.
- ^ a b c d e f Johnston GA (October 2014). "Muscimol as an ionotropic GABA receptor agonist" (PDF). Neurochem Res. 39 (10): 1942–1947. doi:10.1007/s11064-014-1245-y. PMID 24473816.
- ^ a b Zhu W, Huang L, Cheng H, Li N, Zhang B, Dai W, Wu X, Zhang D, Feng W, Li S, Xu H (December 2024). "GABA and its receptors' mechanisms in the treatment of insomnia". Heliyon. 10 (23) e40665. Bibcode:2024Heliy..1040665Z. doi:10.1016/j.heliyon.2024.e40665. PMC 11626785. PMID 39654705.
- ^ a b "γ-Aminobutyric Acid (GABA): Biosynthesis, Role, Commercial Production, and Applications". Studies in Natural Products Chemistry. Vol. 57. Elsevier. 2018. pp. 413–452. doi:10.1016/b978-0-444-64057-4.00013-2. ISBN 978-0-444-64057-4. Retrieved 5 October 2025.
- ^ a b Oketch-Rabah HA, Madden EF, Roe AL, Betz JM (August 2021). "United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA)". Nutrients. 13 (8): 2742. doi:10.3390/nu13082742. PMC 8399837. PMID 34444905.
- ^ a b c Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S (2015). "Neurotransmitters as food supplements: the effects of GABA on brain and behavior". Front Psychol. 6: 1520. doi:10.3389/fpsyg.2015.01520. PMC 4594160. PMID 26500584.
- ^ a b García-Flores E, Farías R (1997). "gamma-Amino-beta-hydroxybutyric acid as add-on therapy in adult patients with severe focal epilepsy". Stereotact Funct Neurosurg. 69 (1-4 Pt 2): 243–246. doi:10.1159/000099882. PMID 9711762.
- ^ Corelli, Federico; Mugnaini, Claudia (2016). "Chemistry of GABAB Receptor Ligands: Focus on Agonists and Antagonists". GABAB Receptor. Cham: Springer International Publishing. pp. 17–32. doi:10.1007/978-3-319-46044-4_2. ISBN 978-3-319-46042-0. Retrieved 5 October 2025.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. ISBN 978-3-88763-075-1. Retrieved 5 October 2025.
- ^ Grbesa B, Vasiljević P, Vlajin L (1967). "Gamibetal (gama-amino-beta-hidroksibuterna kiselina--GABOB) u lecenju epilepsije" [Gamibetal (gamma-amino-beta-hydroxybutyric acid--GABOB in the treatment of epilepsy]]. Med Glas (in Serbian). 21 (3): 75–77. PMID 6082678.
- ^ Michelot D, Melendez-Howell LM (February 2003). "Amanita muscaria: chemistry, biology, toxicology, and ethnomycology" (PDF). Mycol Res. 107 (Pt 2): 131–146. doi:10.1017/s0953756203007305. PMID 12747324.
- ^ a b c Okhovat A, Cruces W, Docampo-Palacios ML, Ray KP, Ramirez GA (2023). "Psychoactive Isoxazoles, Muscimol, and Isoxazole Derivatives from the Amanita (Agaricomycetes) Species: Review of New Trends in Synthesis, Dosage, and Biological Properties". Int J Med Mushrooms. 25 (9): 1–10. doi:10.1615/IntJMedMushrooms.2023049458. PMID 37824402.
- ^ Benkherouf AY, Taina KR, Meera P, Aalto AJ, Li XG, Soini SL, Wallner M, Uusi-Oukari M (April 2019). "Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors". J Neurochem. 149 (1): 41–53. doi:10.1111/jnc.14646. PMC 6438731. PMID 30565258.
- ^ Olsen, Richard W.; Wallner, Martin; Rogawski, Michael A. (2024). "GABAA Receptors, Seizures, and Epilepsy". Jasper's Basic Mechanisms of the Epilepsies. Oxford University PressNew York. pp. 1025–1046. doi:10.1093/med/9780197549469.003.0048. ISBN 978-0-19-754946-9. PMID 39637168.
Muscimol, the psychoactive substance in Amanita muscaria, and its conformationally constrained derivative gaboxadol [4,5,6,7- tetrahydroisoxazolo(5,4-c)pyridin-3-ol; THIP], structurally resemble GABA, and like GABA, they are exceptionally potent as direct activators of extrasynaptic GABAR containing the δ-subunit (Meera et al., 2011; Benkherouf et al., 2019). Indeed, there is drastically reduced high-affinity (6 nM) [3H]muscimol binding in the brains of δ-subunit knockout mice (Mihalek et al., 1999). Low-dose THIP and muscimol are expected to preferentially activate GABAR δ so that the relatively rare GABAR δ receptor subtypes (about 5%–10% of total GABAR) are of particular importance to their biochemical and behavioral actions (Chandra et al., 2006, 2010; Mäkelä et al., 1997; Beaumont et al., 1978).
- ^ a b Stebelska K (August 2013). "Fungal hallucinogens psilocin, ibotenic acid, and muscimol: analytical methods and biologic activities". Ther Drug Monit. 35 (4): 420–442. doi:10.1097/FTD.0b013e31828741a5. PMID 23851905.
- ^ Brimblecombe RW, Pinder RM (1975). "Miscellaneous Hallucinogens". Hallucinogenic Agents. Bristol: Wright-Scientechnica. pp. 196–216. ISBN 978-0-85608-011-1. OCLC 2176880. OL 4850660M.
Waser (1967) describes the effects of self-administration of 10-15 mg. of muscimol as '. . . intense hallucinations as with LSD were missing . . . there resulted considerable disturbances of psychic functions, such as orientation in space and time, visual perception, process of thinking, speech, and some new psychic phenomena of illusions and echo pictures'. Higher doses tended to produce severe intoxication in man, with painful muscular twitching, considerable agitation, and vivid hallucinations. [...] WASER, P. G. (1967), in Ethnopharmacologic Search for Psychoactive Drugs (see BREKHMAN and SAM), pp. 419–439.
- ^ Ott J (1996). "Ibotenic Acid–Muscimol: The Primordial Pangk and Amrta" (PDF). Pharmacotheon: Entheogenic Drugs, Their Plant Sources and History (2 ed.). Natural Products Company. pp. 323–358, 446. ISBN 978-0-9614234-8-3.
- ^ Krogsgaard-Larsen P, Brehm L, Schaumburg K (1981). "Muscimol, a psychoactive constituent of Amanita muscaria, as a medicinal chemical model structure". Acta Chem Scand B. 35 (5): 311–324. doi:10.3891/acta.chem.scand.35b-0311. PMID 6274117.
- ^ Hartwig J, Kendrick J, Ahmad G, Cook J, Matthews DB, Sharma P (2025). "Exploring User Experiences with Amanita muscaria: A Thematic Analysis of Reddit Online Forum Discussions". Subst Use Misuse. 60 (7): 952–961. doi:10.1080/10826084.2025.2476141. PMID 40057818.
- ^ Savickaitė E, Laubner-Sakalauskienė G (2025). "Emerging Risks of Amanita Muscaria: Case Reports on Increasing Consumption and Health Risks". Acta Med Litu. 32 (1): 182–189. doi:10.15388/Amed.2025.32.1.23. PMC 12239171. PMID 40641545.
- ^ Ordak M, Galazka A, Nasierowski T, Muszynska E, Bujalska-Zadrozny M (April 2023). "Reasons, Form of Ingestion and Side Effects Associated with Consumption of Amanita muscaria". Toxics. 11 (4): 383. Bibcode:2023Toxic..11..383O. doi:10.3390/toxics11040383. PMC 10142736. PMID 37112610.
- ^ Dijk DJ, Landolt HP (2019). "Sleep Physiology, Circadian Rhythms, Waking Performance and the Development of Sleep-Wake Therapeutics". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 253: 441–481. doi:10.1007/164_2019_243. ISBN 978-3-030-11270-7. PMID 31254050.
- ^ Wisden W, Yu X, Franks NP (2019). "GABA Receptors and the Pharmacology of Sleep". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 253: 279–304. doi:10.1007/164_2017_56. ISBN 978-3-030-11270-7. PMID 28993837.
- ^ "Merck, Lundbeck scrap insomnia drug after trials". Reuters. 9 August 2007. Retrieved 30 September 2025.
- ^ Miller, R.J. (2013). Drugged: The Science and Culture Behind Psychotropic Drugs. EBL ebooks online. Oxford University Press. ISBN 978-0-19-995798-9. Retrieved 5 October 2025.
- ^ Lundbeck (28 March 2007). "Discontinuation of development program for gaboxadol in insomnia: Teleconference 28 March 2007" (PDF). Archived from the original (PDF) on 17 October 2007.
- ^ Paredes RG, Agmo A (1992). "GABA and behavior: the role of receptor subtypes". Neurosci Biobehav Rev. 16 (2): 145–170. doi:10.1016/s0149-7634(05)80177-0. PMID 1321392.
The mixed GABA-A-GABA-B agonist, progabide, has also shown limited success as an anticonvulsant. Although there are some reports describing clinically useful antiepileptic activity of this compound (197), high doses of progabide are required for significant reduction of symptoms in epileptic patients (187). Moreover, the attenuation of convulsive responses in animal models after progabide administration occurred only at doses that also produced sedation and ataxia (174,287).
- ^ a b Taylor, Charles P. (1990). "GABA receptors and GABAergic synapses as targets for drug development". Drug Development Research. 21 (3): 151–160. doi:10.1002/ddr.430210302. ISSN 0272-4391.
GABA PRODRUGS: Progabide was made as a prodrug of GABA and also has a metabolite that is a potent GABA agonist. It has anticonvulsant effects in several animal models [Bartholini et al., 1985; Morselli et al., 19861, but clinical trials have produced mixed results [Chadwick, 19901. As of this writing, progabide is available only in France for treatment of seizures, and is of continued interest as a pharmacological tool. It is interesting that some of the undesired neurological and behavioral effects of GABA, agonists such as muscimol (myoclonus, psychological disturbances) have not been reported with progabide; this may partly be due to progabide's lower potency relative to muscimol.
- ^ a b Meldrum BS (1989). "GABAergic mechanisms in the pathogenesis and treatment of epilepsy". Br J Clin Pharmacol. 27 Suppl 1 (Suppl 1): 3S – 11S. doi:10.1111/j.1365-2125.1989.tb03454.x. PMC 1379672. PMID 2667605.
Progabide is a GABA receptor agonist acting on both GABAA and GABAB receptors, and is metabolised in the brain to yield SL 75102 (a more potent GABA agonist than progabide) and to GABA itself (Lloyd et al., 1982). It is by no means certain that progabide acts by enhancing GABAergic transmission: in some test systems it mimics the action of phenytoin rather than that of muscimol (Fromm et al., 1985). Progabide is anticonvulsant in a wide range of rodent models of epilepsy (Worms et al., 1982) and has some efficacy in man.
- ^ Shek, Efraim (1994). "Chemical delivery systems and prodrugs of anticonvulsive drugs". Advanced Drug Delivery Reviews. 14 (2–3): 227–241. doi:10.1016/0169-409X(94)90041-8.
Progabide (Fig. 6) is a GABA receptor agonist; however, it is also a pro-drug slowly releasing GABA [45]. The immediate onset of action is explained by the agonist properties of progabide while its ability to release GABA accounts for the duration of action. Chemically, progabide is a Schiff base obtained from ~,-aminobutyramide and a substituted benzophenone. Man and animal metabolize progabide extensively. Only very small amounts of the administered drug can be found in urine [39]. Progabide can convert to the acid analog SL-75102 by hydrolysis, by oxidative deamination, or by transamination. By cleavage of the imine bond, progabide and SL-75102 can release GABA-mide or GABA, respectively. The enzymatic system capable of imine bond cleavage has not been identified. Yet it must be present, because both GABA and GABA-mide are found in the brain after administration of progabide to rats.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. ISBN 978-3-88763-075-1. Retrieved 5 October 2025.
- ^ Perucca E, White HS, Bialer M (September 2023). "New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: II. Treatments in Clinical Development". CNS Drugs. 37 (9): 781–795. doi:10.1007/s40263-023-01025-4. PMC 10501930. PMID 37603261.
Although many of the currently marketed [antiseizure medications (ASMs)] act on the GABA system (Table 1), only three of those, namely vigabatrin, tiagabine and ganaxolone, were rationally designed to exert a GABAergic effect. A fourth rationally designed compound, progabide, was developed in the 1980s as a GABA prodrug but never became established because of disappointing clinical efficacy results and the propensity to cause liver toxicity [101,102,103,104].
- ^ a b c d e Santillo MF, Sprando RL (April 2023). "Picamilon, a γ-aminobutyric acid (GABA) analogue and marketed nootropic, is inactive against 50 biological targets". Basic Clin Pharmacol Toxicol. 132 (4): 355–358. doi:10.1111/bcpt.13836. PMID 36668678.
- ^ Mirzoyan, R. S. (2025). "Significant Achievements of Experimental Pharmacology and Neurology That Were Not Crowned by Success in the Clinic, and Not Only (Review)". Pharmaceutical Chemistry Journal. 59 (1): 1–8. doi:10.1007/s11094-025-03356-6. ISSN 0091-150X. Retrieved 6 October 2025.
- ^ Jędrejko K, Catlin O, Stewart T, Anderson A, Muszyńska B, Catlin DH (August 2023). "Unauthorized ingredients in "nootropic" dietary supplements: A review of the history, pharmacology, prevalence, international regulations, and potential as doping agents". Drug Test Anal. 15 (8): 803–839. doi:10.1002/dta.3529. PMID 37357012.
- ^ Matsuyama K, Yamashita C, Noda A, Goto S, Noda H, Ichimaru Y, Gomita Y (October 1984). "Evaluation of isonicotinoyl-gamma-aminobutyric acid (GABA) and nicotinoyl-GABA as pro-drugs of GABA". Chem Pharm Bull (Tokyo). 32 (10): 4089–4095. doi:10.1248/cpb.32.4089. PMID 6529802.
- ^ Petersen JG, Bergmann R, Møller HA, Jørgensen CG, Nielsen B, Kehler J, Frydenvang K, Kristensen J, Balle T, Jensen AA, Kristiansen U, Frølund B (February 2013). "Synthesis and biological evaluation of 4-(aminomethyl)-1-hydroxypyrazole analogues of muscimol as γ-aminobutyric acid(a) receptor agonists". J Med Chem. 56 (3): 993–1006. doi:10.1021/jm301473k. PMID 23294161.
- ^ Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA, Palmer AA (June 2012). "Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal". J Clin Invest. 122 (6): 2306–2315. doi:10.1172/JCI61319. PMC 3366407. PMID 22585572.
- ^ Gouliaev AH, Senning A (May 1994). "Piracetam and other structurally related nootropics". Brain Res Brain Res Rev. 19 (2): 180–222. doi:10.1016/0165-0173(94)90011-6. PMID 8061686.
- ^ Pluskal T, Weng JK (March 2018). "Natural product modulators of human sensations and mood: molecular mechanisms and therapeutic potential". Chem Soc Rev. 47 (5): 1592–1637. doi:10.1039/c7cs00411g. PMID 28933478.
- ^ Evans RH, Francis AA, Hunt K, Martin MR, Watkins JC (June 1978). "Quisqualamine, a novel gamma-aminobutyric acid (GABA) related depressant amino acid". J Pharm Pharmacol. 30 (6): 364–367. doi:10.1111/j.2042-7158.1978.tb13257.x. PMID 26767.
- ^ Herrero JF (March 1994). "GABAergic activity of quisqualamine and homoquisqualamine in hemisected spinal cord in vitro preparation". Rev Esp Fisiol. 50 (1): 11–17. PMID 7527570.
- ^ Krogsgaard-Larsen P, Frølund B, Jørgensen FS, Schousboe A (August 1994). "GABAA receptor agonists, partial agonists, and antagonists. Design and therapeutic prospects". J Med Chem. 37 (16): 2489–2505. doi:10.1021/jm00042a001. PMID 8057295.
- ^ Frolovskii, V. A.; Studnev, Yu. N.; Garibova, T. L.; Voronina, T. A. (2004). "Some aspects in the search for anticonvulsants (a review)". Pharmaceutical Chemistry Journal. 38 (9): 467–479. doi:10.1007/s11094-004-0001-z. ISSN 0091-150X. Retrieved 5 October 2025.
- ^ Krogsgaard-Larsen P (December 1981). "gamma-Aminobutyric acid agonists, antagonists, and uptake inhibitors. Design and therapeutic aspects". J Med Chem. 24 (12): 1377–1383. doi:10.1021/jm00144a001. PMID 6118436.
- ^ Crider AM, Tita TT, Wood JD, Hinko CN (November 1982). "Esters of nipecotic and isonipecotic acids as potential anticonvulsants". J Pharm Sci. 71 (11): 1214–1219. doi:10.1002/jps.2600711108. PMID 7175711.
Isonipecotic acid (Ib) was shown to be a potent and specific y-aminobutyric acid agonist in the [3H]y-aminobutyric acid-binding assay procedure (13,14). As in the case of nipecotic acid, isonipecotic acid was also too polar to penetrate the blood-brain barrier.
- ^ Sarup A, Larsson OM, Schousboe A (August 2003). "GABA transporters and GABA-transaminase as drug targets". Curr Drug Targets CNS Neurol Disord. 2 (4): 269–277. doi:10.2174/1568007033482788. PMID 12871037.
- ^ Czuczwar SJ, Patsalos PN (2001). "The new generation of GABA enhancers. Potential in the treatment of epilepsy". CNS Drugs. 15 (5): 339–350. doi:10.2165/00023210-200115050-00001. PMID 11475940.
- ^ Kowalczyk P, Kulig K (2014). "GABA system as a target for new drugs". Curr Med Chem. 21 (28): 3294–3309. doi:10.2174/0929867321666140601202158. PMID 24934345.
- ^ Madsen KK, Larsson OM, Schousboe A (2008). "Regulation of excitation by GABA neurotransmission: focus on metabolism and transport". Results Probl Cell Differ. Results and Problems in Cell Differentiation. 44: 201–221. doi:10.1007/400_2007_036. ISBN 978-3-540-72601-2. PMID 17579816.