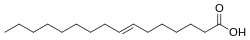
Elaidic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(E)-octadec-9-enoic acid
| |
| Other names
(E)-9-octadecenoic acid
(9E)-octadecenoic acid trans-9-octadecenoic acid 18:1 trans-9 C18:1 trans-9 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.642 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C 18H 34O 2 | |
| Molar mass | 282.46 g/mol |
| Appearance | colorless waxy solid |
| Density | 0.8734 g/cm3 |
| Melting point | 45 °C (113 °F) |
| −204.8·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Elaidic acid is a chemical compound with the formula C
18H
34O
2, specifically the fatty acid with structural formula HOOC−(CH2)7−CH=CH−(CH2)7−CH3, with the double bond (between carbon atoms 9 and 10) in trans configuration. It is a colorless solid. Its salts and esters are called elaidates.
Elaidic acid is an unsaturated trans fatty acid, with code C18:1 trans-9. This compound has attracted attention because it is a major trans fat found in hydrogenated vegetable oils, and trans fats have been implicated in heart disease.[1]
It is the trans isomer of oleic acid. The name of the elaidinization reaction comes from elaidic acid.
Etymology
[edit]The name elaidic comes from the Ancient Greek word ἔλαιον (élaion), meaning oil.
Occurrence and bioactivity
[edit]Elaidic acid occurs mostly in industrial hydrogenation of polyunsaturated fatty acids.[2] It's also present in small amounts in goat and cow milk (very roughly 0.1% of the fatty acids)[3] and in some meats.[4]
Elaidic acid increases plasma cholesterylester transfer protein (CETP) activity which lowers HDL cholesterol.[5]
See also
[edit]References
[edit]- ^ Tardy, Anne-Laure; Morio, Béatrice; Chardigny, Jean-Michel; Malpuech-Brugère, Corinne (2011). "Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases". Nutrition Research Reviews. 24 (1): 111–7. doi:10.1017/S0954422411000011. PMID 21320382.
- ^ Wendeu-Foyet, Gaëlle; Bellicha, Alice; Chajès, Véronique; Huybrechts, Inge; Bard, Jean-Marie; Debras, Charlotte; Srour, Bernard; Sellem, Laury; Fezeu, Léopold K.; Julia, Chantal; Kesse-Guyot, Emmanuelle; Agaësse, Cédric; Druesne-Pecollo, Nathalie; Galan, Pilar; Hercberg, Serge (2023). "Different Types of Industry-Produced and Ruminant Trans Fatty Acid Intake and Risk of Type 2 Diabetes: Findings From the NutriNet-Santé Prospective Cohort". Diabetes Care. 46 (2): 321–330. doi:10.2337/dc22-0900. PMID 36542554. S2CID 255041911.
- ^ Alonso L, Fontecha J, Lozada L, Fraga MJ, Juárez M (1999). "Fatty acid composition of caprine milk: major, branched-chain, and trans fatty acids". J. Dairy Sci. 82 (5): 878–84. doi:10.3168/jds.S0022-0302(99)75306-3. hdl:10261/113439. PMID 10342226.
- ^ Stillwell, William (2016). "Chapter 23. Membranes and Human Health". An Introduction to Biological Membranes – Composition, Structure and Function (2 ed.). Elsevier. doi:10.1016/B978-0-444-63772-7.00023-3.
- ^ Abbey M, Nestel PJ (1994). "Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet". Atherosclerosis. 106 (1): 99–107. doi:10.1016/0021-9150(94)90086-8. PMID 8018112.