Arsenic tribromide
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Arsenic tribromide | |
| Systematic IUPAC name
Tribromoarsane | |
| Other names
Arsenic(III) bromide
Arsenous bromide, Arsenicum Bromatum, Tribromoarsine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.143 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AsBr3 | |
| Molar mass | 314.634 g/mol |
| Appearance | white to pale yellow crystalline solid |
| Density | 3.54 g/cm3 |
| Melting point | 31.1 °C (88.0 °F; 304.2 K) |
| Boiling point | 221 °C (430 °F; 494 K) |
| soluble, partial hydrolysis indicated by fumes | |
| −106.0·10−6 cm3/mol | |
Refractive index (nD)
|
2.3 |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3[1] |
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute][1] |
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)][1] |
| Related compounds | |
Related compounds
|
Phosphorus tribromide arsenic trichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
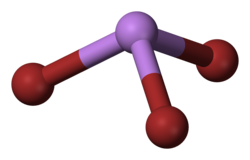
Arsenic tribromide is an inorganic compound with the formula As Br3, it is a bromide of arsenic. Arsenic is a chemical element that has the symbol As and atomic number 33. This pyramidal molecule is the only known binary arsenic bromide. AsBr3 is noteworthy for its very high refractive index of approximately 2.3. It also has a very high diamagnetic susceptibility.[2] The compound exists as colourless deliquescent crystals that fume in moist air.
Preparation
[edit]Arsenic tribromide is optimally prepared by treating arsenic(III) oxide with hot concentrated hydrobromic acid.[3]
- As2O3 + 6 HBr → 2 AsBr3 + 3 3 H2O
It can also be prepared by the direct bromination of arsenic powder. Alternatively, arsenic(III) oxide can be treated with a mixture of elemental sulfur and bromine:[4]
- 2 As2O3 + 3 S + 6 Br2 → 4 AsBr3 + 3 SO2
Arsenic tribromide hydrolyzes readily. It is soluble in hydrocarbons.
Bromides of arsenic
[edit]AsBr5 is not known, although the corresponding phosphorus compound PBr5 is well characterized. AsBr3 is the parent for a series of hypervalent anionic bromoarsenates including [As2Br8]2−, [As2Br9]3−, and [As3Br12]3−.[5]
Organoarsenic bromides (CH3)2AsBr and (CH3)AsBr2 are formed efficiently by the copper-catalyzed reaction of methyl bromide with hot arsenic metal. This synthesis is similar to the direct process used for the synthesis of methyl chlorosilanes.[citation needed]
Safety
[edit]Arsenic tribromide is highly toxic. It is a carcinogen and a teratogen.[citation needed]
References
[edit]- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH).
- ^ CRC handbook of Chemistry and Physics, CRC Press
- ^ Tanaka, Susumu; Konishi, Masafumi; Imoto, Hiroaki; Nakamura, Yuma; Ishida, Masatoshi; Furuta, Hiroyuki; Naka, Kensuke (2020). "Fundamental Study on Arsenic(III) Halides (AsX3; X = Br, I) toward the Construction of C 3-Symmetrical Monodentate Arsenic Ligands". Inorganic Chemistry. 59 (14): 9587–9593. doi:10.1021/acs.inorgchem.0c00598. PMID 32515950.
- ^ P. W. Schenk (1963). "Arsenic (III) Bromide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY,NY: Academic Press. p. 597.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.